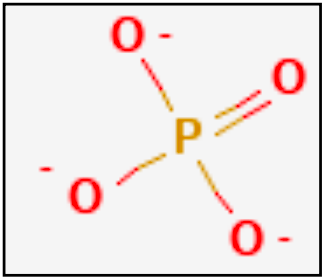
Phosphates play a central role in biological systems. This situation shows that they also played an important role in the emergence of life on Earth. The wide and varied biological roles of phosphates are testament to their value in controlling chemistry and building strong structures in an aqueous environment.[1] A phosphate is a salt formed either from a single PO4 tetrahedron or by the condensation of a large number of PO4 anions and also has an anionic presence.[2] Compounds based on the presence of an anionic phosphorus (O4P-3) consisting of an almost regular tetrahedral arrangement of four oxygen atoms surrounding a central phosphorus atom are described as the most common form of phosphates.[2] (Fig.1: Chemical Structure Depiction and 2D Structure of Phosphate.[4])
Phosphate materials can be divided into 4 classes: monophosphates, condensed (dense phosphorus) phosphates, phosphates with additional anion groups, and heteropolyphosphates.[9]
The compounds formed by [PO4] 3- with a phosphorus atom in the center and 4 oxygen atoms in a regular tetrahedral structure are called monophosphate. These salts, known as orthophosphate for a long time, are called monophosphate today. However, both uses are still frequently encountered in the scientific literature. Monophosphates form a large family in phosphate chemistry. Because they have been well known for a long time and are very stable. In all other families of phosphates, the phosphoric anion contains P-O-P bonds that are sensitive to hydrolysis.[9]
In the term condensed phosphates, salts containing condensed phosphoric anions are used. Such a complete definition of anion can be given by saying the phosphoric anion containing one or more P-O-P bonds as condensed phosphoric anion. Many different processes can be applied to the structure of these bonds. Condensed phosphates are divided into three subgroups. The first group is polyphosphates. Their general formula is [PnO3n + 1] (n + 2) -. “n” is the number of phosphorus atoms in the anionic formula. Depending on the value of n in the first expression of this condensation, they are often called oligo-phosphates. n = 2 profphosphate [P2O7] 4-, n = 3 tripolyphosphate [P3O10] 5-, n = 4 tetra polyphosphate [P4O13] 6-. The second group is cyclophosphates. Depending on the structure of the cyclic anions, the second type of condensation is formed from rings where the corners of the tetrahedral PO4 unit are shared, led by the anionic formula [PnO3n] n-. These rings are characterized for n = 3, 4, 5, 6, 7, 8, 9, 10, 11, 12. Last group are ultraphosphates. This type of condensation occurs in condensed anions with anionic structure, long chain anions, and all condensed phosphates where P2O5 is richer than the richest members of the two classes of ring anions. From this definition the formulas of anions are given as n [PO3] – + mP2O5 or [P (2m + n) O (5m-3n)] n-.[9]
Phosphate compounds containing anions such as O2-, OH-, F-, Cl-, Br-, I-, NO3-, BO3- are known as phosphates with additional anion groups. The compounds Ln7O6 (BO3) (PO4) and MnNa3 (PO4) (CO3) are examples of phosphate compounds of this type.[9]
In chemistry, phosphates are salts or esters of phosphoric acid, either of mineral and organic origin or they are industrial products. Mineral phosphates are obtained from rock phosphates. For this, rocks are first melted in hot water loaded with carbon dioxide, then phosphate is precipitated in various ways. Organic origin phosphates occur as follows. Plants first consume the phosphorus in the soil formed from rocks. Aquatic animals that eat these plants and obtain phosphorus from seawater store phosphorus in their bodies. Large phosphate deposits are formed as a result of the fecal cadavers and organic matter they have accumulated by decomposition. These types of deposits are phosphate deposits of organic origin. Phosphates that are formed by the change of phosphates, that is, from the deformation of ammonium phosphate and iron, are industrial phosphates.[8]
3 types of phosphoric acid create 3 types of phosphate. These are in order: Metaphosphate (P03), Pyrophosphate (PiA), orthophosphate (P0).[8]
Salts consisting of these are: sodium metaphosphate ( NaPOa ), sodium pyrophosphate ( Na4P207 ) ve sodium orthophosphate ( Na3P04 ).[8]
Phosphates can be formed by combining phosphorus pentoxide (P205) with metal oxides or hydroxides. If metal, metal oxides or hydroxides combine with suitable acids, phosphates are formed.[8]
Na20 + PB05->2NaPQ3
2Na20 + P205.-*Na4P207
3Na O + PA.*->2Na3P04
Orthophosphates are the most important phosphates. These are the salts of phosphoric acid represented by the formula H3PO4. However, since orthophosphoric acid is a trivalent acid, three types of phosphate can be formed.[8]
- NaOH+H3PO4-*HB0+NaH2P04 Monosodium phosphate
- NaOH + NaH2P04 -> H20 + Na2HP04 Disodium phosphate
- NaOH + Naf HP04-> HaO + Na3P04 Trisodium phosphate .
Since polyphosphates are dehydrated forms obtained by water coming out of more than one orthophosphate molecule, they undergo hydrolysis in the aquatic environment over time and return to their ortho state.

The rate of this transformation is a function of temperature, and the speed increases as the temperature approaches the boiling point. The rate of hydrolysis can also be increased by decreasing the pH. Hydrolysis of complex phosphates; it also happens through bacterial enzymes. For this reason, the conversion rate in clean water is less than wastewater.
There are tertiary phosphates or their derivatives carrying only PO4 ions in nature. These are simple or compound salts made by 2-valuable (Ca, Mg) metals such as Mg and Ca. Phosphorite found in North Africa is a tricalcium phosphate and can be represented as Ca5 (PO4) 3Cl or Cas (PO4) 3F. Another phosphate found in nature. They are piles of bird droppings that take the name Guano, accumulated over thousands of years. This is found in some Pacific Islands. Its composition is close to phosphorite.
Phosphate has various physiological roles. And it includes preservation of cell membrane integrity as phospholipids, synthesis of DNA and RNA, energy conservation and transfer as phosphorylated intermediates, and regulation of proteins by phosphorylation / dephosphorylation. And, DNA is linked by phosphate.[6](Fig.2: Our Dna linked with phosphate.[6])

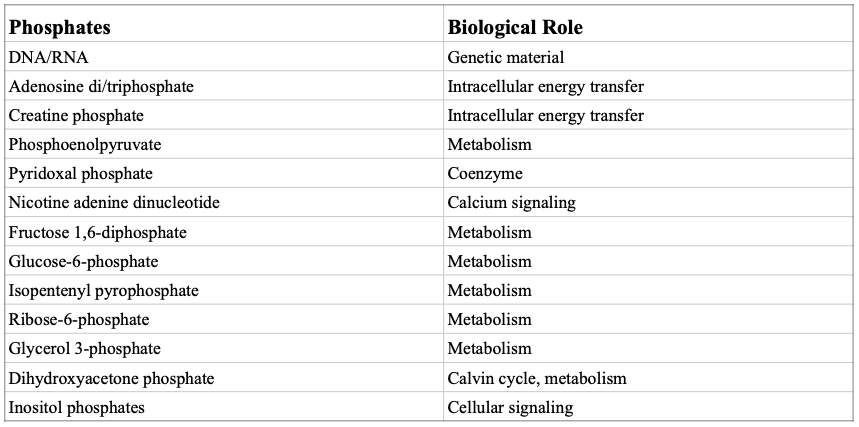
Phosphate also contributes to skeletal mineralization in vertebrates.[7] Dephosphorylation is catalyzed by phosphatases. More than 2000 chemical reactions in living cells use phosphate. And for the effectiveness of such biochemical reactions, an optimal balance of intracellular and extracellular phosphate is needed.Homoeostatic control of phosphate is one of the most delicate biological regulations because many intracellular pathways utilize phosphate ions for important cellular reactions.[5] Phosphates play a central role in the transference of phosphoryl groups from one entity to another, in biosynthesis, control of secondary messengers, regulation of protein function, and preservation of the integrity of genetic material. Both DNA and RNA are phosphate esters like many intermediate metabolites.[2] (Table1: Some examples of biologically related phosphate.[2])
Phosphate has many uses around the world. And its importance is increasing day by day. First of all, the most widely used phosphates in cleaners and detergents are sodium phosphates.[9] In addition, monocalic and monosodic phosphates are used in baking powder production. It is also used to form a protective layer around insoluble phosphate salts on metal surfaces, to polish metal surfaces, and to electro polish on phosphoric acid baths.[8]
Phosphate compounds are used in different places, in phosphoric acid carbonated beverages, calcium phosphate as a food supplement, and monocalcium phosphate monohydrate and sodium aluminum phosphate as acid disintegrants. As another use, both disodium phosphate and sodium tripolyphosphate are used in human treatments. Also; phosphates improve the freshness of processed fish such as canned seafood. And dicalcium phosphate dihydrate is often used as a brightening material in toothpastes.[9]
On the other hand; phosphorus is an essential nutrient for the body. And it is regularly consumed through food. However, it is found in the body as phosphate because after consumption, phosphorus is usually bound to oxygen. Both organic and inorganic forms of phosphate are found in regularly consumed foods such as meat, fish, eggs, milk / dairy products and vegetables.[5]

In general, the daily phosphate need of the body is covered by intestinal absorption from the consumed foods. And the serum phosphate level is precisely maintained by the renal excretion of phosphate.[5]
More than 80% of total phosphate is found in bones and teeth in the form of apatite.The remaining phosphate is mostly found in internal organs and skeletal muscle. Apart from that, it is found in very small amounts in extracellular fluids. About 30% of the total cellular phosphate is stored in the endoplasmic reticulum and is also used in the phosphorylation of various proteins. The remainder is found in cellular phosphate nucleus, Golgi complex and lysosomes. The transport of phosphate into and out of the cell according to the needs of the body is called a complex process. And the exact molecular mechanisms of such delicate transport have not yet been clearly elucidated. It should be noted that the cells also use phosphate to transport cellular energy through the formation of ATP by oxidative phosphorylation. Additionally, the synthesis of glucose and triacylglycerol (triglyceride) uses phosphate to form glucose 6- phosphate and glycerol 3-phosphate, respectively.[5]

Phosphate deficiency or excess can have a negative impact on survival. Therefore, organisms have developed systems to maintain phosphate homeostasis. Organisms have duties to maintain in order to maintain phosphate homeostasis. Organisms must detect changes in environmental and internal phosphate levels and adapt to them. [7] Dietary phosphate deficiency, often due to malnutrition, can lead to hypophosphatemia. But it just doesn’t cause that. It can also disrupt the bone mineralization process. And as a result can lead to the development of rickets.[5]
We can see, thanks to modern biochemical evidence, that phosphates are very likely to play an important role in the chemical origin of life. In addition, the primary role that phosphates play in biology may indicate that accumulation in local environments (eg ponds, lakes or craters) plays an important role in defining the environment. All these show that phosphates play an important role in the emergence of life on earth.
REFERENCES
[1] A Chemist’s Perspective on the Role of Phosphorus at the Origins of Life: Christian Fernández-García, AdamJ.Coggins and MatthewW.Powner (Department of Chemistry, University College London, 20 Gordon Street, London WC1H 0AJ, UK) Life 2017, 7(3), 31; https://doi.org/10.3390/life7030031.
[2] Why nature really chose phosphate: Shina C. L. Kamerlin1, Pankaz K. Sharma2, Ram B. Prasad2, Arieh Warshel2,* 1Department of Cell and Molecular Biology (ICM), Uppsala Biomedical Centre, Uppsala University, Box 596, S-751 24 Uppsala, Sweden 2Department of Chemistry (SGM 418), University of Southern California, 3620 McClintock Avenue, Los Angeles, CA 90089, USA
[3] Westheimer, F. Why nature chose phosphates. (Science, New Series, Vol. 235, No. 4793. ((Mar. 6, 1987), pp. 1173-1178.)
[4] https://pubchem.ncbi.nlm.nih.gov/compound/Phosphate
[5] Phosphate toxicity: new insights into an old problem: M. Shawkat RAZZAQUE, (Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA 02115, U.S.A.)
[6] Organic Chemistry with a Biological Emphasis (Soderberg) by Tim Soderberg, Overview of Phosphate Groups. (Emeritus Associate Professor of Chemistry at University of Minnesota Morris)
[7] Phosphate as a Signaling Molecule and Its Sensing Mechanism, Toshimi Michigami, Masanobu Kawai, Miwa Yamazaki, and Keiichi Ozono.
[8] Berker, E . (1972). Fosfat Kimyası, Kullanılış Alanları ve Super fosfat . Bilimsel Madencilik Dergisi , 11 (4) , 59-62 . Retrieved from “https://dergipark.org.tr/tr/pub/madencilik/issue/32669/362541”.
[9] Bazı Metal İçeren Boratlı, Fosfatlı, ve Borfosfatlı Bileşiklerin Sentezi ve Yapısal Karakterizasyonu, Doktora Tezi, Berna TEKİN, (T.C. Balıkesir Üniversitesi Fen Bilimleri Enstitüsü Kimya Anabilim Dalı)
 Genetikçe Everything about science.
Genetikçe Everything about science.